Difference between revisions of "Documentation/Nightly/Modules/PkModeling"
(4.1 -> Nightly) |
Tag: 2017 source edit |
||
| (27 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| + | <noinclude>{{documentation/versioncheck}}</noinclude> | ||
<!-- ---------------------------- --> | <!-- ---------------------------- --> | ||
{{documentation/{{documentation/version}}/module-header}} | {{documentation/{{documentation/version}}/module-header}} | ||
| Line 9: | Line 10: | ||
Extension: [[Documentation/{{documentation/version}}/Extensions/PkModeling|PkModeling]]<br> | Extension: [[Documentation/{{documentation/version}}/Extensions/PkModeling|PkModeling]]<br> | ||
Acknowledgments: | Acknowledgments: | ||
| − | This work is part of the National Alliance for Medical Image Computing (NA-MIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research.<br> | + | This work is part of the National Alliance for Medical Image Computing (NA-MIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, and by National Cancer Institute as part of the Quantitative Imaging Network initiative (U01CA151261) and QIICR (U24CA180918).<br> |
Implementation of the pharmacokinetics modeling was contributed by Yingxuan Zhu and Jim Miller from GE Research.<br> | Implementation of the pharmacokinetics modeling was contributed by Yingxuan Zhu and Jim Miller from GE Research.<br> | ||
| − | Author: Yingxuan Zhu, Jim Miller ({{collaborator|name|ge}})<br> | + | Author: Yingxuan Zhu (while at GE), Andrey Fedorov (SPL), John Evans (MGH), Jim Miller ({{collaborator|name|ge}})<br> |
| − | Contact: | + | Contact: Jim Miller, <email>millerjv@ge.com</email><br> |
{{documentation/{{documentation/version}}/module-introduction-row}} | {{documentation/{{documentation/version}}/module-introduction-row}} | ||
{{documentation/{{documentation/version}}/module-introduction-logo-gallery | {{documentation/{{documentation/version}}/module-introduction-logo-gallery | ||
|{{collaborator|logo|ge}}|{{collaborator|longname|ge}} | |{{collaborator|logo|ge}}|{{collaborator|longname|ge}} | ||
|{{collaborator|logo|namic}}|{{collaborator|longname|namic}} | |{{collaborator|logo|namic}}|{{collaborator|longname|namic}} | ||
| + | |{{collaborator|logo|qiicr}}|{{collaborator|longname|qiicr}} | ||
}} | }} | ||
| Line 28: | Line 30: | ||
# Converts signal intensities to concentration values. The concentration values are used to calculate quantitative parameters. | # Converts signal intensities to concentration values. The concentration values are used to calculate quantitative parameters. | ||
# Calculates quantitative parameters from concentration values. These parameters include: | # Calculates quantitative parameters from concentration values. These parameters include: | ||
| − | ;Ktrans: Volume transfer constant between blood plasma and EES (extracellular-extravascular space) at each voxel | + | ;Ktrans: Volume transfer constant between blood plasma and EES (extracellular-extravascular space) at each voxel (min^-1) |
;Ve: Fractional volume for extracellular space at each voxel | ;Ve: Fractional volume for extracellular space at each voxel | ||
;MaxSlope: Maximum slope in the time series curve of each voxel | ;MaxSlope: Maximum slope in the time series curve of each voxel | ||
| Line 37: | Line 39: | ||
<!-- ---------------------------- --> | <!-- ---------------------------- --> | ||
{{documentation/{{documentation/version}}/module-section|Use Cases}} | {{documentation/{{documentation/version}}/module-section|Use Cases}} | ||
| + | * estimation of quantitative perfusion parameters from DCE MRI | ||
| + | * treatment response evaluation | ||
| + | * breast, prostate, brain DCE MRI analysis | ||
{| | {| | ||
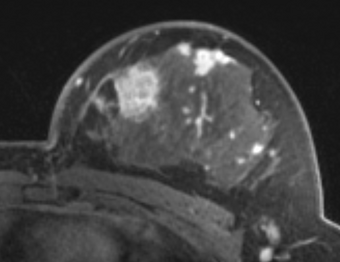
| + | |[[Image:BC1_DCE.png|thumb|340px|Sample frame from a breast DCE MRI dataset (one of the datasets presented in a study by Huang et al. [6]]] | ||
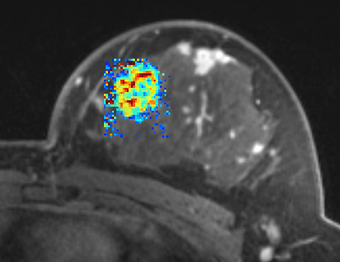
| + | |[[Image:BC1_Ktrans_map_ROI.png|thumb|340px|Ktrans map result of PK modeling using population AIF (Parker et al., [5])]] | ||
| + | |- | ||
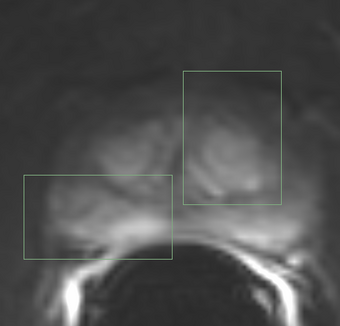
| + | |[[Image:QIN-PROSTATE-2_DCE.png|thumb|340px|Sample frame from a prostate DCE MRI dataset (QIN-PROSTATE-002 from TCIA QIN-PROSTATE collection)]] | ||
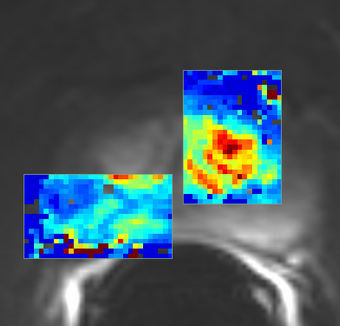
| + | |[[Image:QIN-PROSTATE-2_Ktrans_map_ROI.png|thumb|340px|Ktrans map result of PK modeling using population AIF (Parker et al., [5])]] | ||
|} | |} | ||
<!-- ---------------------------- --> | <!-- ---------------------------- --> | ||
{{documentation/{{documentation/version}}/module-section|Tutorials}} | {{documentation/{{documentation/version}}/module-section|Tutorials}} | ||
| + | |||
| + | * "Exploration and Study of MultiVolume Image Data using 3D Slicer" by Meysam Torabi, Brigham and Women's Hospital: https://github.com/QIICR/PkModeling/files/3875977/MultiVolumeExplorer_Meysam_SNR-April2013-v4.pdf | ||
| + | * "3D Slicer for DCE-MRI Image Analysis" by Madeline Carr, University of Wollongong Australia: https://github.com/QIICR/PkModeling/files/3870911/DCE.and.3D.Slicer.Tutorial.pdf | ||
<!-- ---------------------------- --> | <!-- ---------------------------- --> | ||
| Line 49: | Line 62: | ||
|[[Image:PkModelingUI061912.png|thumb|340px|PkModeling]] | |[[Image:PkModelingUI061912.png|thumb|340px|PkModeling]] | ||
| | | | ||
| + | * '''PkModeling Parameters''': | ||
| + | ** T1 Blood Value (milliseconds) | ||
| + | ** T1 Tissue Value (milliseconds) (default value is the published value for prostate tissue estimated in healthy individuals (see Ref. de Bazelaire et al.) | ||
| + | ** r1 Relaxivity Value of the contrast agent, L x mol^(-1) x s^(-1). This value is contrast agent specific. Default setting of 0.0039 corresponds to Gd-DPTA (Magnevist) at 3T, see Ref. Pintaske et al. You will need to adjust this setting based on the magnet signal strength and contrast agent. | ||
| + | ** Hematocrit Value. Volume percentage of red blood cells in blood. | ||
| + | ** AUC Time Interval Value: Time interval for AUC calculation | ||
| + | ** Use Population AIF: A mean AIF is calculated from a functional form instead of from the input using the aifMask or a prescribed AIF. See Ref. Parker et al. | ||
* '''IO''' | * '''IO''' | ||
| − | ** '''Input | + | ** '''Input 4D Image''': 4D DCE-MRI data |
| − | ** ''' | + | ** '''T1 Map Image''': T1 Map |
| − | * ''' | + | ** '''AIF Mask Image''': Mask designating the location of the arterial input function (AIF). AIF can either be calculated from the input using the aifMask, prescribed directly in concentration units using the prescribedAIF option, or via a population AIF. |
| − | ** ''' | + | ** '''Prescribed AIF''': Prescribed arterial input function (AIF). AIF can either be calculated from the input using the aifMask option, via a population AIF, or can be prescribed directly in concentration units using the prescribedAIF option. An example of how a prescribed AIF can be defined is in [[File:AIF_example.mcsv.zip|this example .mcsv file]] (unzip before importing into Slicer!). Note that the x column corresponds to timestamps in seconds, and the y column is the contrast agent concentration (NOT image signal intensity). |
| − | ** | + | ** '''Output Ktrans image''': volume transfer constant between blood plasma and extravascular extracellular space (EES) (min^-1) |
| − | ** | + | ** '''Output ve image''': volume of EES per unit volume of tissue |
| − | *** | + | ** '''Output fpv image''': (or v_p) blood plasma volume per unit of tissue |
| − | ** | + | ** '''Output maximum slope image''': maximum slope of the signal intensity curve between any two consecutive timepoints |
| − | ** | + | ** '''Output AUC image''': area under the curve in the first 90 seconds |
| − | ** ''' | + | * '''Advanced options''': |
| − | *** TR Value: Repetition time | + | ** '''BAT Calculation Mode''': PeakGradient (Default) or UseConstantBAT |
| − | + | ** '''Constant BAT''': Constant Bolus Arrival Time Index (frame number) | |
| − | + | ** '''Output R-squared goodness of fit image''': each pixel will be initialized to a value between 0 and 1 characterizing the goodness of fit. Larger values correspond to a better fit (see [http://en.wikipedia.org/wiki/Coefficient_of_determination R^2 measure description]) | |
| − | * | + | ** '''Output Bolus Arrival Time Image''': the bolus arrival time calculated at each pixel |
| + | |} | ||
| + | |||
| + | The following acquisition parameters should be available in the NRRD header of the input data (if you are analyzing a DICOM time series, they will typically be extracted from the DICOM data): | ||
| + | * TR Value: Repetition time (milliseconds) | ||
| + | * TE Value: Echo time (milliseconds) | ||
| + | * FA Value: Flip angle (degrees) | ||
| + | * Timestamps for the dynamic series (in milliseconds) | ||
| − | + | Here is an example how this information is represented in the NRRD header: | |
| + | <pre> | ||
| + | MultiVolume.DICOM.EchoTime:=2.93 | ||
| + | MultiVolume.DICOM.FlipAngle:=10 | ||
| + | MultiVolume.DICOM.RepetitionTime:=6.13 | ||
| + | MultiVolume.FrameIdentifyingDICOMTagName:=AcquisitionTime | ||
| + | MultiVolume.FrameIdentifyingDICOMTagUnits:=ms | ||
| + | </pre> | ||
| + | |||
| + | '''Auto generated documentation of the parameters''' | ||
| + | |||
| + | {{documentation/{{documentation/version}}/module-parametersdescription}} | ||
<!-- ---------------------------- --> | <!-- ---------------------------- --> | ||
| Line 74: | Line 112: | ||
<!-- ---------------------------- --> | <!-- ---------------------------- --> | ||
{{documentation/{{documentation/version}}/module-section|References}} | {{documentation/{{documentation/version}}/module-section|References}} | ||
| − | * Knopp MV, Giesel FL, Marcos H et al: Dynamic contrast-enhanced magnetic resonance imaging in oncology. Top Magn Reson Imaging, 2001; 12:301-308. | + | * [1] Knopp MV, Giesel FL, Marcos H et al: Dynamic contrast-enhanced magnetic resonance imaging in oncology. Top Magn Reson Imaging, 2001; 12:301-308. |
| − | * Rijpkema M, Kaanders JHAM, Joosten FBM et al: Method for quantitative mapping of dynamic MRI contrast agent uptake in human tumors. J Magn Reson Imaging 2001; 14:457-463. | + | * [2] Rijpkema M, Kaanders JHAM, Joosten FBM et al: Method for quantitative mapping of dynamic MRI contrast agent uptake in human tumors. J Magn Reson Imaging 2001; 14:457-463. |
| − | + | * [3] de Bazelaire, C.M., et al., MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology, 2004. 230(3): p. 652-9. | |
| + | * [4] Pintaske J, Martirosian P, Graf H, Erb G, Lodemann K-P, Claussen CD, Schick F. Relaxivity of Gadopentetate Dimeglumine (Magnevist), Gadobutrol (Gadovist), and Gadobenate Dimeglumine (MultiHance) in human blood plasma at 0.2, 1.5, and 3 Tesla. Investigative radiology. 2006 March;41(3):213–21. | ||
| + | * [5] Parker GJ, Roberts C, Macdonald A, Buonaccorsi GA, Cheung S, Buckley DL, Jackson A, Watson Y, Davies K, Jayson GC. Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magnetic Resonance in Medicine, 2006 Nov; 56(5):993-1000. | ||
| + | * [6] Huang, W., Li, X., Chen, Y., Li, X., Chang, M.-C., Oborski, M. J., … Kalpathy-Cramer, J. (2014). Variations of dynamic contrast-enhanced magnetic resonance imaging in evaluation of breast cancer therapy response: a multicenter data analysis challenge. Translational Oncology, 7(1), 153–66. doi:10.1593/tlo.13838 http://dx.doi.org/10.1593/tlo.13838 | ||
| + | * [7] Tofts, P. S., Brix, G., Buckley, D. L., Evelhoch, J. L., Henderson, E., Knopp, M. V, … Weisskoff, R. M. (1999). Estimating kinetic parameters from Contrast-Enhanced T 1 -Weighted MRI of a Diffusable Tracer : Standardized Quantities and Symbols. J Magn Reson Imaging, 10(3), 223–232. | ||
<!-- ---------------------------- --> | <!-- ---------------------------- --> | ||
{{documentation/{{documentation/version}}/module-section|Information for Developers}} | {{documentation/{{documentation/version}}/module-section|Information for Developers}} | ||
{{documentation/{{documentation/version}}/module-developerinfo}} | {{documentation/{{documentation/version}}/module-developerinfo}} | ||
| + | Source code: https://github.com/millerjv/PkModeling | ||
<!-- ---------------------------- --> | <!-- ---------------------------- --> | ||
{{documentation/{{documentation/version}}/module-footer}} | {{documentation/{{documentation/version}}/module-footer}} | ||
[[Category:Documentation/{{documentation/version}}/Modules/Quantification]] | [[Category:Documentation/{{documentation/version}}/Modules/Quantification]] | ||
<!-- ---------------------------- --> | <!-- ---------------------------- --> | ||
| + | . | ||
Latest revision as of 17:43, 21 November 2019
Home < Documentation < Nightly < Modules < PkModeling
|
For the latest Slicer documentation, visit the read-the-docs. |
Introduction and Acknowledgements
|
Extension: PkModeling | |||||||
|
Module Description
|
PkModeling (Pharmacokinetics Modeling) calculates quantitative parameters from Dynamic Contrast Enhanced DCE-MRI images. This module performs two operations:
|
Use Cases
- estimation of quantitative perfusion parameters from DCE MRI
- treatment response evaluation
- breast, prostate, brain DCE MRI analysis
Tutorials
- "Exploration and Study of MultiVolume Image Data using 3D Slicer" by Meysam Torabi, Brigham and Women's Hospital: https://github.com/QIICR/PkModeling/files/3875977/MultiVolumeExplorer_Meysam_SNR-April2013-v4.pdf
- "3D Slicer for DCE-MRI Image Analysis" by Madeline Carr, University of Wollongong Australia: https://github.com/QIICR/PkModeling/files/3870911/DCE.and.3D.Slicer.Tutorial.pdf
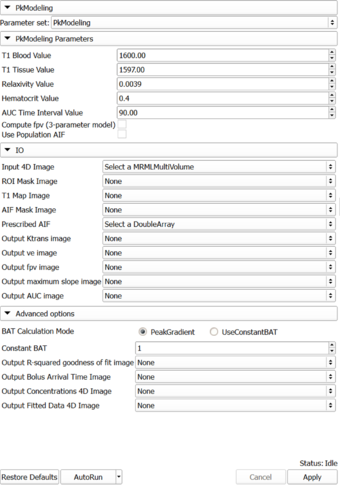
Panels and their use
|
The following acquisition parameters should be available in the NRRD header of the input data (if you are analyzing a DICOM time series, they will typically be extracted from the DICOM data):
- TR Value: Repetition time (milliseconds)
- TE Value: Echo time (milliseconds)
- FA Value: Flip angle (degrees)
- Timestamps for the dynamic series (in milliseconds)
Here is an example how this information is represented in the NRRD header:
MultiVolume.DICOM.EchoTime:=2.93 MultiVolume.DICOM.FlipAngle:=10 MultiVolume.DICOM.RepetitionTime:=6.13 MultiVolume.FrameIdentifyingDICOMTagName:=AcquisitionTime MultiVolume.FrameIdentifyingDICOMTagUnits:=ms
Auto generated documentation of the parameters
Parameters:
- PkModeling Parameters: Parameters used for PkModeling
- T1 Blood Value (T1PreBloodValue): T1 value for blood.
- T1 Tissue Value (T1PreTissueValue): T1 value for tissue.
- Relaxivity Value (RelaxivityValue): Contrast agent relaxivity value. Default value corresponds to Gd-DPTA (Magnevist) at 3T.
- Signal Gradient Threshold Value (S0GradValue): Signal Gradient Threshold value.
- F Tolerance Value (FTolerance): Tolerance on function value for establishing convergence.
- G Tolerance Value (GTolerance): Tolerance on gradient value for establishing convergence.
- X Tolerance Value (XTolerance): Tolerance on parameter values for establishing convergence.
- Epsilon Value (Epsilon): Epsilon value.
- Maximum number of iterations (MaxIter): Maximum number of iterations in optimizing the parameter estimation at each voxel.
- Hematocrit Value (Hematocrit): Hematocrit value, volume percentage of red blood cells in blood.
- AUC Time Interval Value (AUCTimeInterval): AUC Time Interval value.
- Compute fpv (3-parameter model) (ComputeFpv): Enable estimation of fractional plasma volume at each voxel.
- Use Population AIF (UsePopulationAIF): Use generic population AIF.
- IO: Input/output parameters
- Input 4D Image (InputFourDImageFileName): Input 4D Image (txyz)
- ROI Mask Image (ROIMaskFileName): (Optional) Mask designating the voxels (non-zero) where the model should be fit. If not specified, the model will be fit to all voxels in the image.
- T1 Map Image (T1MapFileName): (Optional) Volume defining T1 value for each voxel.
- AIF Mask Image (AIFMaskFileName): Mask designating the location of the arterial input function (AIF). AIF can be calculated from a generic population AIF, the input using the aifMask or can be prescribed directly in concentration units using the prescribedAIF option.
- Prescribed AIF (PrescribedAIFFileName): Prescribed arterial input function (AIF). AIF can either be calculated from the input using the aifMask option or can be prescribed directly in concentration units using the prescribedAIF option.
- Output Ktrans image (OutputKtransFileName): Output volume transfer constant between blood plasma and EES (extracellular-extravascular space) at each voxel.
- Output ve image (OutputVeFileName): Output fractional volume for extracellular space at each voxel.
- Output fpv image (OutputFpvFileName): Output fractional plasma volume at each voxel.
- Output maximum slope image (OutputMaxSlopeFileName): Output maximum slope in the time series curve of each voxel.
- Output AUC image (OutputAUCFileName): Output area under the curve (AUC) of each voxel, measured from bolus arrival time to the end time of interval, normalized by the AUC of the AIF.
- Advanced options: Debugging and advanced research options.
- BAT Calculation Mode (BATCalculationMode): Determine how to calculate bolus arrival time.
- Constant BAT (ConstantBAT): Constant Bolus Arrival Time index(frame number).
- Output R-squared goodness of fit image (OutputRSquaredFileName): R-squared goodness of fit of the model to each voxel. Setting this output changes the returned images. When this output is not used, the output parameter volumes only contain the voxels where good fits were obtained. When this output is set, the parameter volumes for every voxel is output and may be masked by the R-squared volume in a separate module.
- Output Bolus Arrival Time Image (OutputBolusArrivalTimeImageFileName): Output Per-Pixel Bolus Arrival Time (xyz)
- Output Concentrations 4D Image (OutputConcentrationsImageFileName): Output Concentrations 4D Image (txyz)
- Output Fitted Data 4D Image (OutputFittedDataImageFileName): Output Fitted Data 4D Image (txyz)
- Output Diagnostics Image (OutputOptimizerDiagnosticsImageFileName): Output map with the optimizer diagnostics. The code is encoded in 2 hex numbers. Lower 4 bits encode the optimizer errors are as follows:\n0: OIOIOI -- failure in leastsquares function\n1: OIOIOI -- lmdif dodgy input\n2: converged to ftol\n3: converged to xtol\n4: converged nicely\n5: converged via gtol\n6: too many iterations\n7: ftol is too small. no further reduction in the sum of squares is possible.\n8: xtol is too small. no further improvement in the approximate solution x is possible.\n9: gtol is too small. Fx is orthogonal to the columns of the jacobian to machine precision.\n10: OIOIOI: unknown info code from lmder.\n11: optimizer failed, but diagnostics string was not recognized.\nUpper 4 bits encode other non-optimizer errors or notifications:\n16 (0x10): Ktrans was clamped to [0..5].\n32 (0x20): Ve was clamped to [0..1].\n48 (0x30): BAT detection failed.\n64 (0x40): BAT at the voxel was less than AIF BAT.\n
List of parameters generated transforming this XML file using this XSL file. To update the URL of the XML file, edit this page.
Similar Modules
References
- [1] Knopp MV, Giesel FL, Marcos H et al: Dynamic contrast-enhanced magnetic resonance imaging in oncology. Top Magn Reson Imaging, 2001; 12:301-308.
- [2] Rijpkema M, Kaanders JHAM, Joosten FBM et al: Method for quantitative mapping of dynamic MRI contrast agent uptake in human tumors. J Magn Reson Imaging 2001; 14:457-463.
- [3] de Bazelaire, C.M., et al., MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology, 2004. 230(3): p. 652-9.
- [4] Pintaske J, Martirosian P, Graf H, Erb G, Lodemann K-P, Claussen CD, Schick F. Relaxivity of Gadopentetate Dimeglumine (Magnevist), Gadobutrol (Gadovist), and Gadobenate Dimeglumine (MultiHance) in human blood plasma at 0.2, 1.5, and 3 Tesla. Investigative radiology. 2006 March;41(3):213–21.
- [5] Parker GJ, Roberts C, Macdonald A, Buonaccorsi GA, Cheung S, Buckley DL, Jackson A, Watson Y, Davies K, Jayson GC. Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magnetic Resonance in Medicine, 2006 Nov; 56(5):993-1000.
- [6] Huang, W., Li, X., Chen, Y., Li, X., Chang, M.-C., Oborski, M. J., … Kalpathy-Cramer, J. (2014). Variations of dynamic contrast-enhanced magnetic resonance imaging in evaluation of breast cancer therapy response: a multicenter data analysis challenge. Translational Oncology, 7(1), 153–66. doi:10.1593/tlo.13838 http://dx.doi.org/10.1593/tlo.13838
- [7] Tofts, P. S., Brix, G., Buckley, D. L., Evelhoch, J. L., Henderson, E., Knopp, M. V, … Weisskoff, R. M. (1999). Estimating kinetic parameters from Contrast-Enhanced T 1 -Weighted MRI of a Diffusable Tracer : Standardized Quantities and Symbols. J Magn Reson Imaging, 10(3), 223–232.
Information for Developers
| Section under construction. |
Source code: https://github.com/millerjv/PkModeling .